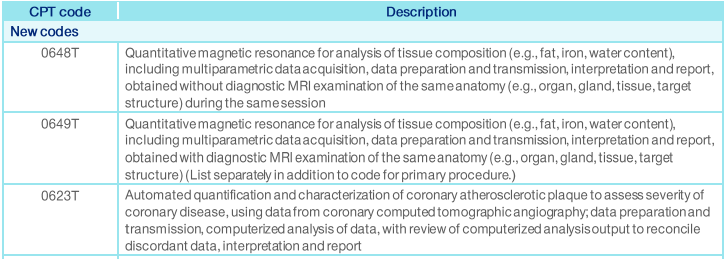
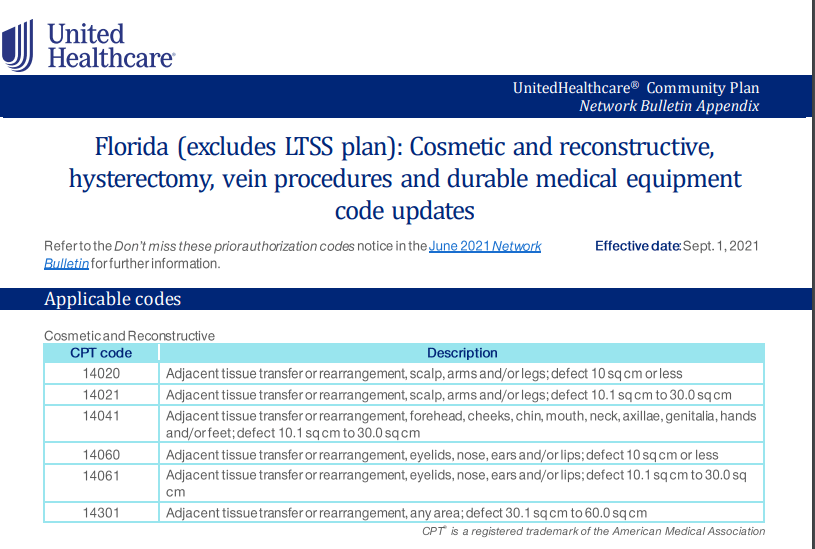
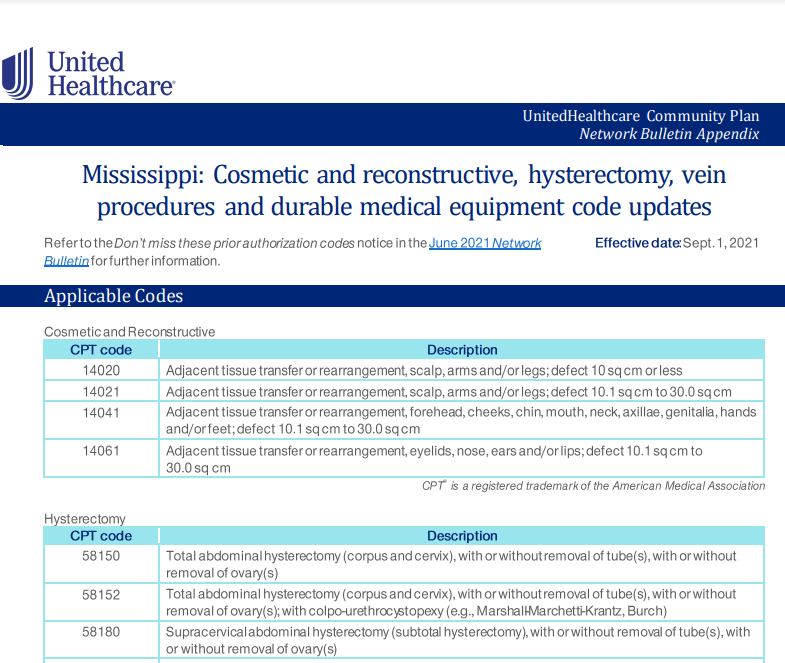
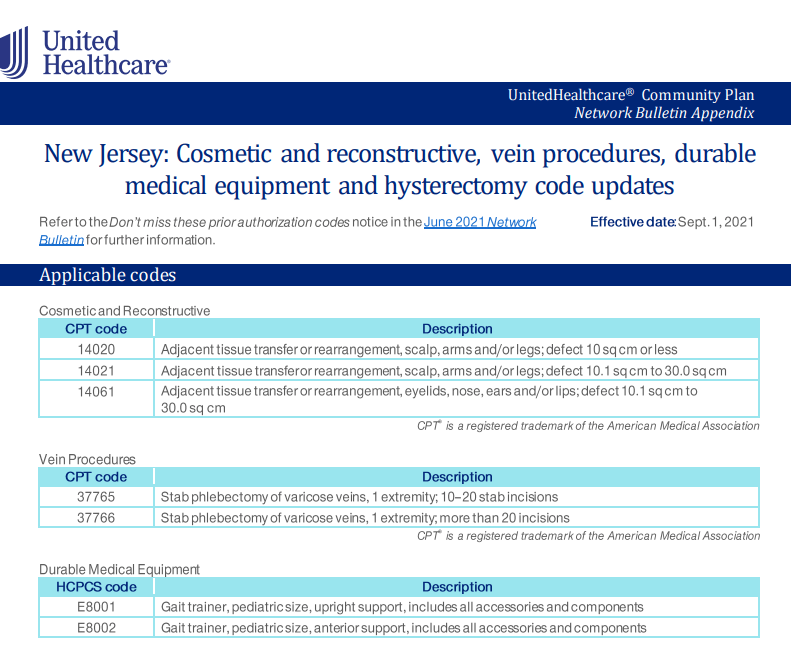
This policy change will take affect on 10/01/2021 and will apply to UHC NY Community Health patients that have a primary Cancer diagnosis.
Any active prior authorizations requested via the former process will remain in place.
Effective Oct. 1, 2021, Optum will manage our prior authorization requests for outpatient injectable cancer therapies, including:
- Chemotherapy and biologic therapy
- Colony stimulating factors
- Denosumab
Requesting prior authorization
Submit prior authorization requests online at UHCprovider.com > Prior Authorization and Notification > OncologyOpens in a new windowopen_in_new. Sign into the UnitedHealthcare Provider Portal using your One Healthcare ID and select the Prior Authorization and Notification tool. Once you are in the tool, select Oncology and answer the questions about the service type, member type and state.
Other Medications requiring Prior Authorization:
Effective Oct. 1, 2021, UHC NY Community Plan will require prior authorization for the following therapeutic radiopharmaceuticals:
- Lutetium Lu 177 (Lutathera®)
- Radium RA-233 dichloride (Xofigo®)
- Iobenguane I 131 (Azedra®)
- All therapeutic radiopharmaceuticals that have not yet received an assigned code and will be billed under a miscellaneous Healthcare Common Procedure Coding System (HCPCS) code
Those therapeutic radiopharmaceuticals can be billed under the following HCPCS codes:
- A9590-Iodine I-131, iobenguane, 1 mCi
- A9513 Lutetium Lu 177, dotatate, therapeutic, 1 mCi
- A9606 Radium RA-223 dichloride, therapeutic, per microcurie
- A9699 Radiopharmaceutical, therapeutic, not otherwise classified
On Oct. 1, 2021, UHC NY Community Plan also require prior authorization for the following 5 anti-emetic codes for members with a cancer diagnosis. Prior authorization requirements for outpatient injectable chemotherapy are not affected.
- J0185 aprepitant, 1 mg
- J1453 fosaprepitant, 1 mg
- J1454 fosnetupitant 235 mg and palonosetron 0.25 mg
- J1627 granisetron, extended-release, 0.1 mg
- J2469 palonosetron HCl, 25 mcg
Preferred products for anti-emetics include Emend®, Kytril® and Zofran®.
NY Community Health Plan coverage criteria for Medical Benefit Drug Policy titled Anti-Emetics for Oncology:
If a member receives therapeutic radiopharmaceuticals and/or anti-emetics for a cancer diagnosis in an outpatient setting between July 1, 2021, and Sept. 30, 2021, you don’t need to request prior authorization until you administer a new therapeutic radiopharmaceutical drug or anti-emetic. We’ll authorize the therapeutic radiopharmaceutical drug and/or anti-emetic the member was receiving prior to Oct. 1, 2021. The authorization will be effective until Sept. 30, 2022.
Other Medical Benefit Drug Policies are used for coverage reviews for cancer therapies and cancer supportive drugs, such as the colony-stimulating factors:
- Oncology Medication Clinical Coverage Policy
- White Blood Cell Colony Stimulating Factors
These policies include coverage criteria for non-preferred products. If a member receives a non-preferred product in an outpatient setting between July 1, 2021, and Sept. 31, 2021, you don’t need to request a new prior authorization for these products. Those existing authorizations will be honored through their end date.
All policies are available at UHCprovider.com > Policies and Protocols > Community Plan Policies > Medical & Drug Policies and Coverage Determination Guidelines for Community Plan.
Prior authorization will continue to be required for:
- Chemotherapy and biologic therapy injectable drugs (J9000–J9999), Leucovorin (J0640) and Levoleucovorin (J0641 and J0642)
- Chemotherapy and biologic therapy injectable drugs that have a Q code
- Chemotherapy and biologic therapy injectable drugs that have not yet received an assigned code and will be billed under a miscellaneous Healthcare Common Procedure Coding System (HCPCS) code
- Colony-stimulating factors:
- J1442 Filgrastim (Neupogen®)
- J1447 Tbo-filgrastim (Granix®)
- J2505 Pegfilgrastim (Neulasta®)
- J2820 Sargramostim (Leukine®)
- Q5101 Filgrastim, biosimilar (Zarxio®)
- Q5108 Pegfilgrastim-jmdb (Fulphila™)
- Q5110 Filgrastim-aafi (Nivestym™)
- Q5120 Pegfilgrastim-bmez, biosimilar, (Ziextenzo®)
- Q5111 Pegfilgrastim-cbqv, biosimilar (Udenyca™)
- Q5122 Pegfilgrastim-apgf (Nyvepria™)
- Colony-stimulating factors that have not yet received an assigned code and will be billed under a temporary or miscellaneous HCPCS code will require prior authorization
- Denosumab (Brand names Xgeva® and Prolia®): J0897 prior authorization will be required when adding a new injectable chemotherapy drug or cancer therapy to an existing regimen
Recent Posts: